How to Determine Which Atom Is Larger
Larger and heavier atoms and molecules exhibit stronger dispersion forces than smaller and lighter ones. If the steric number is 3 sp2.
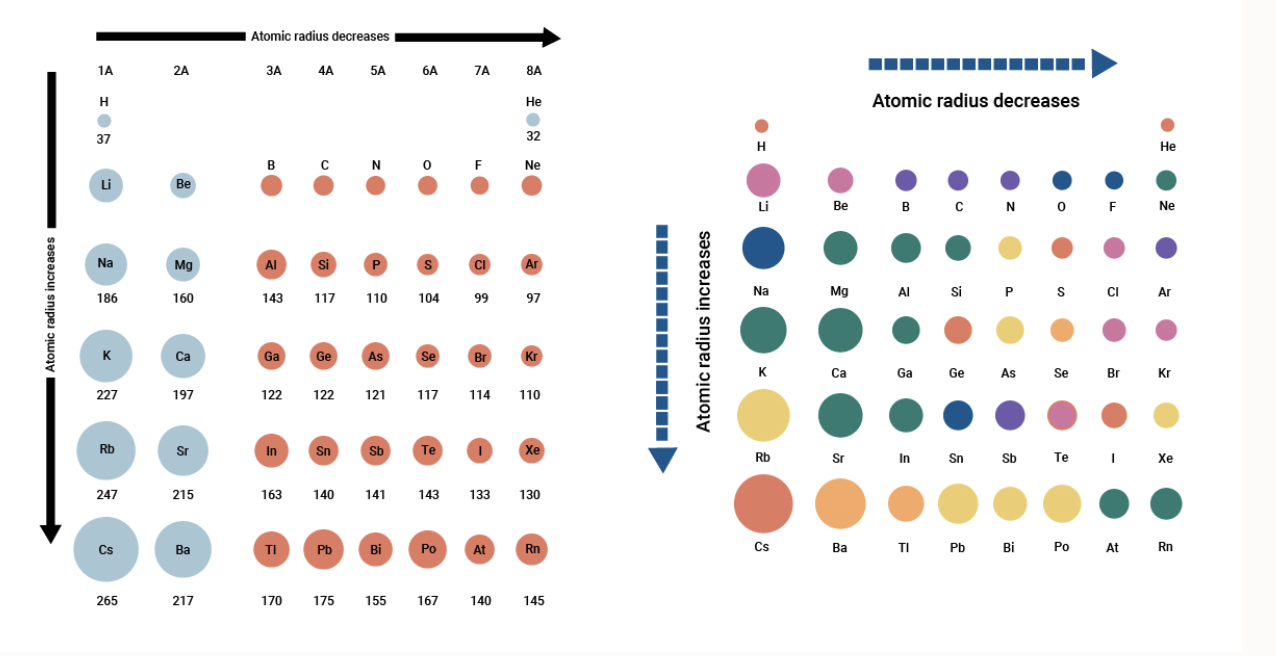
The usual periodic trend for atomic size places larger atoms at the left of a row and towards the bottom of a column on the periodic table.

. Recall that -1 means that one electron has been added to the chlorine atom. Use the periodic table to determine which elements are likely to have a larger atomic radius than silicon Si. Check all that apply.
In general the bigger the atom the greater the ability to lose electrons. If the molecule is bigger then the surface area is larger which results in a bigger LDF. Size of atom can be determine by periodic table and Valence electron.
A Although nitrogen N and phosphorus P are in the same group nitrogen is more. I dont see a text but i am gonna assume this orbit the curved path usually elliptical described by a planet satellite spaceship etc around a celestial body as the sun. The size of the atoms is determined by the size of their orbitals.
Why arent francium atoms the biggest. The larger the quantum number n is the larger the orbital is which increases the size of the atom. Phosphorus belongs to the third period.
The covalent radius of a chlorine atom for example is half the distance between the nuclei of the atoms in a Cl 2 molecule. For Cl the number of protons are fewer than the number of electrons. The valence shell the outer peel of the atom is largest in atoms at the bottom of the periodic table.
In other words the carbon atom is 57 as large as the iron atom. Earlier we proved that directly. Atomic number Z 15 and nitrogen belongs to the second period atomic number Z 7 and hence the size of phosphorus is larger than nitrogen.
They need more energy to escape to the gas phase so the larger molecule has the higher boiling point. Suppose you have two substances both of which happen to interact through LDF. If the steric number is 2 sp.
C2 SN 3 three atoms connected therefore it is sp2. The atomic radius of a chemical element is a measure of the size of its atoms. They are less tightly held and can more easily form temporary dipoles.
Cations are smaller than the corresponding neutral atoms since the valence electrons which are furthest away from the nucleus are lost. Removal of electrons results in an ion that is smaller than the parent element. Aluminum Al carbon C sulfur S tin Sn 2 See answers.
For the alkaline earth cations Mg2 and Ca2 you have a third Period cation versus a fourth Period cation. C1 SN 3 three atoms connected therefore it is sp2. Another thing to consider is the mass of the molecule.
Now lets determine the proton-electron ratio for Cl and Cl For neutral Cl the number of protons are equal to the number of electrons. 0071 nm versus 0124 nm 2. Clearly the calcium ion should be larger.
Nuclear charge increases going left to right across table rows so the largest atoms should be found on the left edge of the table. Francium is an alkali metal in group 1IA. In a larger atom or molecule the valence electrons are on average farther from the nuclei than in a smaller atom or molecule.
All alkali metals have one valence electron. Ionic radius is determined by measuring the atom in a crystal lattice. The atomic radius of the carbon atom is much less than that for iron.
The greater the shielding the greater the ability to lose electrons. See answer 1 Best Answer Copy The number of full or partially filled electron shells determines the size of an atom. Use the periodic table to determine which elements are likely to have a larger atomic radius than silicon Si Get the answers you need now.
Why is francium so reactive. Atoms further down the periodic table are larger because they have more shells of electrons Atoms farther to the right on the table are smaller because th. Atomic size is difficult to measurebecause it has no definite boundary.
Every atom is different in its number of protons its mass and its size. The size of an atom can be estimated by measuring the distance between adjacent atoms in a covalent compound. The valence should expand and thus anions should have a larger radius than their parent atoms.
A larger molecule is more polarizable which is an attraction that keeps the molecules together. The size of the outermost shell depends on how many protons in the nucleus. Compare sodium nitrate and rubidium nitrate in terms of molecular weight and boiling point.
If the atom is larger is is easier has a greater chance of hitting the object with a know size the opposite is true also. So now lets go back to our molecule and determine the hybridization states for all the atoms. The covalent radii of the main group elements are given in the figure below.
In general anions are larger than the corresponding neutral atom since adding electrons increases the number of electron-electron repulsion interactions that take place. Atomic radii vary in a predictable manner across the periodic table. Addition of electrons results in an ion that is larger than the parent atom.
It represents the mean distance from the nucleus to the boundary of the surrounding cloud of electrons. Atomic size is the distance from the nucleus to the valence shell where the valence electrons are located. This leads us to the conclusion that the mass of the molecule is proportional to the boiling point.
If the steric number is 4 it is sp3. The effective nuclear charge of fluorine is. Assuming atoms have a spherical shape the radius of the sphere describes the size of the atom.
The usual periodic trend for atomic size places larger atoms at the left of a row and towards the bottom of a column on the periodic table. I DONT WANT MY ANSWER COLLAPSED BOT FOOD. Scientists have developed techniques to determine the boundary of an atom by using other atoms of the same element.
39 Related Question Answers Found Why does electronegativity increase up. From a simple APF calculation you should know that BCC has a larger total volume of interstitial space than FCC. A low nuclear charge means that electrons can wander further on average from the nucleus.
Also Know how do you find the largest atom. Elements make up everything and they combine to make compounds.

Pin Auf Chapter 2 The Chemistry Of Life

Ions Isotopes And Bohr Models Atom Review Lab Ngss Aligned Teaching Chemistry Teaching Middle School Science Teaching Science
How Would You Determine Which Element Has A Larger Or Smaller Atomic Radius Quora

This Photo Displays A Decomposition Synthesis And Exchange Reactions In A Decomposition Reaction A Large Molecule Is Bro Molecules Synthesis Photo Displays

Pin By John O Brien On Physics Science Chemistry Physics And Mathematics Science Facts

50 Periodic Table Webquest Worksheet Answers Chessmuseum Template Library Text Features Worksheet Webquest Syllable Worksheet

Csr Reporting 120 Sustainability Report Infographics Infographic Sustainability Csr

Atoms Molecules And Ions Worksheets Chemistry Worksheets Chemistry Classroom Chemistry Interactive Notebook

Periodic Trends Introductory Chemistry 1st Canadian Edition Chemistry Periodic Table Blocks Ionization Energy

Chemistry Of Teflon Cookware Chemistry Chemistry Help Chemical Science

Question Video Identifying Which Element Has An Atomic Radius Larger Than Aluminum Nagwa

Problem Solving Summary Sheet Chemistry Topics Chemistry Chemistry Lessons Problem Solving

How Would You Determine Which Element Has A Larger Or Smaller Atomic Radius Quora

Physics Tuition Atomic Theory Cool Science Experiments

Organic System Plan Template Saferbrowser Image Search Results Organic Chemistry Reactions Organic Chemistry Chemistry Worksheets

Eighth Grade Lesson Scale Model Of An Atom Atom Model Earth Science Classroom Middle School Literacy


Comments
Post a Comment